LANS Clinical Research Consulting
Clinical /Quality/ Regulatory
Services
Industries
Pharmaceutical
Medical Device
Biologics
Clinical, Quality, Regulatory Associate
Responsible for the design, implementation, and monitoring of clinical trials. Ensure that clinical trials are conducted in accordance with standard operating procedures, Good Clinical Practice (GCP), and guidelines meeting quality and regulatory standards. Protecting the safety and well-being of study participants.
Welcome to LANS Clinical Research. We are here to provide you with all of your clinical research needs:Services
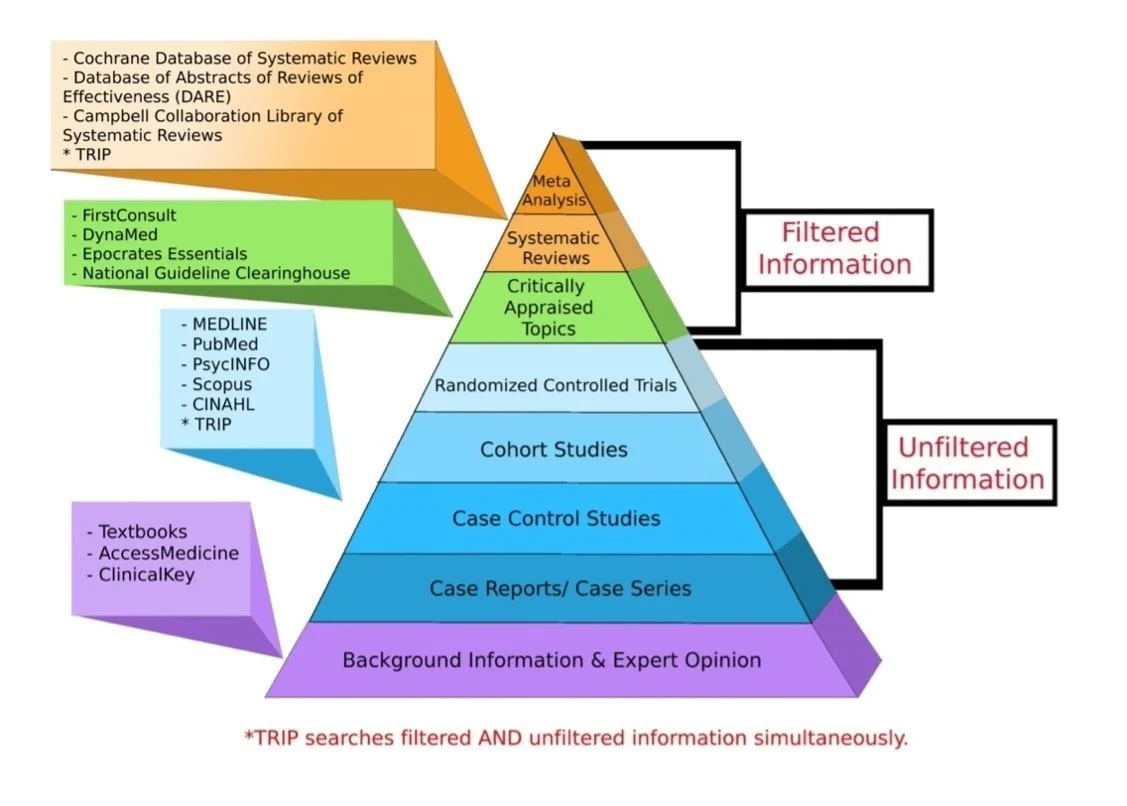
Clinical Research - Protocol & CSR Writing/Randomization/Blinding/Cohort StudiesData Management - Data Collection/ Analysis/Adverse Event ReportingCase Report Form Design
Regulatory-GCP/FDA/ICH Guidelines/IRBStudy Management - SOPs/Subject Recruitment/Informed Consent
Associate Director
Provides leadership and guidance to study teams, including mentoring and managing staff to enhance team performance and development. Develop and maintain strong relationships with key stakeholders, including external vendors, clinical sites, and regulatory bodies.
Director
Directs overall strategy and delivery of deliverables. Analyze, develop, and execute resource management modeling, inclusive of operational, financial, and administrative models..
Book an appointment
For more information or an appointment please email us at lansclinicalresearch@gmail.com.